Blood Flow to the Gi Tract Is Approximately What Percentage of the Total Cardiac Output?

Major factors influencing cardiac output – heart charge per unit and stroke volume, both of which are also variable.[1]
Cardiac output (CO), also known as heart output denoted by the symbols , or ,[two] is a term used in cardiac physiology that describes the volume of blood being pumped by both ventricles of the middle, per unit of measurement time (normally measured per minute). Cardiac output (CO) is the product of the heart charge per unit (Hour), i.e. the number of heartbeats per minute (bpm), and the stroke volume (SV), which is the book of blood pumped from the left ventricle per crush; thus giving the formula:
- CO = Hour × SV[3]
Values for cardiac output are usually denoted equally L/min. For a good for you individual weighing seventy kg, the cardiac output at residual averages nearly 5 Fifty/min; bold a heart charge per unit of 70 beats/min, the stroke volume would be approximately 70 ml.
Considering cardiac output is related to the quantity of blood delivered to various parts of the torso, information technology is an important component of how efficiently the center can meet the body'southward demands for the maintenance of acceptable tissue perfusion. Torso tissues crave continuous oxygen delivery which requires the sustained send of oxygen to the tissues by systemic circulation of oxygenated claret at an adequate pressure from the left ventricle of the heart via the aorta and arteries. Oxygen delivery (DO2 mL/min) is the resultant of blood menses (cardiac output CO) times the blood oxygen content (CaO2). Mathematically this is calculated as follows: oxygen delivery = cardiac output × arterial oxygen content, giving the formula
- DO2 = CO × CaO2.[4]
With a resting cardiac output of five litre min−1, a 'normal' oxygen delivery is around 997.5 ml/min. The amount/per centum of the circulated oxygen consumed (VO2) per minute through metabolism varies depending on the activity level but at rest is circa 25% of the DOii. Concrete exercise requires a college than resting-level of oxygen consumption to support increased muscle activity. In the instance of middle failure, bodily CO may exist insufficient to support even elementary activities of daily living; nor tin it increment sufficiently to run into the higher metabolic demands stemming from fifty-fifty moderate exercise.
Cardiac output is a global blood flow parameter of interest in hemodynamics, the study of the catamenia of claret. The factors affecting stroke volume and middle rate too affect cardiac output. The effigy at the correct margin illustrates this dependency and lists some of these factors. A detailed hierarchical illustration is provided in a subsequent figure.
There are many methods of measuring CO, both invasively and non-invasively; each has advantages and drawbacks as described below.
Definition [edit]
The office of the center is to drive blood through the circulatory system in a cycle that delivers oxygen, nutrients and chemicals to the body'south cells and removes cellular waste product. Because it pumps out whatever blood comes back into it from the venous organization, the quantity of blood returning to the center effectively determines the quantity of blood the heart pumps out – its cardiac output, Q. Cardiac output is classically defined alongside stroke volume (SV) and the heart rate (60 minutes) every bit:[ citation needed ]
-
(one)
In standardizing what CO values are considered to be within normal range independent of the size of the subject'due south trunk, the accepted convention is to further index equation (ane) using trunk surface area (BSA), giving rise to the Cardiac index (CI). This is detailed in equation (ii) below.
Measurement [edit]
There are a number of clinical methods to measure cardiac output, ranging from direct intracardiac catheterization to not-invasive measurement of the arterial pulse. Each method has advantages and drawbacks. Relative comparison is limited by the absence of a widely accepted "gilded standard" measurement. Cardiac output tin can also be afflicted significantly by the phase of respiration – intra-thoracic pressure changes influence diastolic filling and therefore cardiac output. This is especially important during mechanical ventilation, in which cardiac output tin vary by up to 50% across a single respiratory cycle.[ citation needed ] Cardiac output should therefore be measured at evenly spaced points over a single cycle or averaged over several cycles.[ commendation needed ]
Invasive methods are well accustomed, but there is increasing testify that these methods are neither accurate nor effective in guiding therapy. Consequently, the focus on development of non-invasive methods is growing.[v] [6] [7]
Doppler ultrasound [edit]
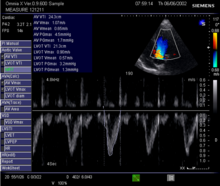
Doppler betoken in the left ventricular outflow tract: Velocity Fourth dimension Integral (VTI)
This method uses ultrasound and the Doppler effect to measure cardiac output. The blood velocity through the heart causes a Doppler shift in the frequency of the returning ultrasound waves. This shift can then be used to calculate menstruum velocity and volume, and finer cardiac output, using the post-obit equations:[ commendation needed ]
where:
- CSA is the valve orifice cross sectional area,
- r is the valve radius, and,
- VTI is the velocity time integral of the trace of the Doppler menstruation profile.
Being non-invasive, accurate and inexpensive, Doppler ultrasound is a routine role of clinical ultrasound; information technology has loftier levels of reliability and reproducibility, and has been in clinical use since the 1960s.[ citation needed ]
Echocardiography [edit]
Echocardiography is a non-invasive method of quantifying cardiac output using ultrasound. Two-dimensional (2D) ultrasound and Doppler measurements are used together to calculate cardiac output. 2nd measurement of the diameter (d) of the aortic annulus allows adding of the flow cross-sectional area (CSA), which is so multiplied by the VTI of the Doppler flow profile across the aortic valve to determine the flow book per beat out (stroke volume, SV). The issue is then multiplied by the middle charge per unit (60 minutes) to obtain cardiac output. Although used in clinical medicine, information technology has a wide exam-retest variability.[8] It is said to crave all-encompassing grooming and skill, simply the exact steps needed to achieve clinically acceptable precision have never been disclosed. 2d measurement of the aortic valve diameter is ane source of noise; others are beat-to-vanquish variation in stroke volume and subtle differences in probe position. An culling that is non necessarily more reproducible is the measurement of the pulmonary valve to calculate right-sided CO. Although information technology is in wide full general use, the technique is time-consuming and is limited by the reproducibility of its component elements. In the manner used in clinical practise, precision of SV and CO is of the lodge of ±20%.[ commendation needed ]
Transcutaneous [edit]
Ultrasonic Cardiac Output Monitor (USCOM) uses continuous wave Doppler to mensurate the Doppler flow contour VTI. It uses anthropometry to calculate aortic and pulmonary valve diameters and CSAs, allowing correct-sided and left-sided Q measurements. In comparison to the echocardiographic method, USCOM significantly improves reproducibility and increases sensitivity of the detection of changes in menstruum. Existent-time, automatic tracing of the Doppler flow profile allows beat-to-vanquish right-sided and left-sided Q measurements, simplifying operation and reducing the time of acquisition compared to conventional echocardiography. USCOM has been validated from 0.12 L/min to eighteen.seven L/min[nine] in new-born babies,[10] children[11] and adults.[12] The method can be applied with equal accuracy to patients of all ages for the development of physiologically rational haemodynamic protocols. USCOM is the merely method of cardiac output measurement to have achieved equivalent accuracy to the implantable catamenia probe.[thirteen] This accuracy has ensured loftier levels of clinical use in conditions including sepsis, heart failure and hypertension.[xiv] [15] [16]
Transoesophageal [edit]

A transoesophageal echocardiogram probe.
The Transoesophageal Doppler includes 2 chief technologies; transoesophageal echocardiogram—which is primarily used for diagnostic purposes, and oesophageal Doppler monitoring—which is primarily used for the clinical monitoring of cardiac output. The latter uses continuous moving ridge Doppler to measure out claret velocity in the descending thoracic aorta. An ultrasound probe is inserted either orally or nasally into the oesophagus to mid-thoracic level, at which indicate the oesophagus lies alongside the descending thoracic aorta. Because the transducer is close to the blood flow, the point is clear. The probe may require re-focussing to ensure an optimal indicate. This method has adept validation, is widely used for fluid management during surgery with evidence for improved patient outcome,[17] [eighteen] [19] [20] [21] [22] [23] [24] and has been recommended past the UK's National Institute for Health and Clinical Excellence (Nice).[25] Oesophageal Doppler monitoring measures the velocity of blood and not true Q, therefore relies on a nomogram[26] based on patient historic period, height and weight to convert the measured velocity into stroke volume and cardiac output. This method generally requires patient sedation and is accustomed for employ in both adults and children.[ citation needed ]
Pulse pressure methods [edit]
Pulse pressure (PP) methods measure the pressure in an artery over time to derive a waveform and use this information to summate cardiac operation. Even so, any measure from the avenue includes changes in force per unit area associated with changes in arterial office, for case compliance and impedance. Physiological or therapeutic changes in vessel diameter are assumed to reflect changes in Q. PP methods measure out the combined performance of the eye and the blood vessels, thus limiting their awarding for measurement of Q. This can exist partially compensated for by intermittent calibration of the waveform to another Q measurement method and so monitoring the PP waveform. Ideally, the PP waveform should be calibrated on a trounce-to-beat basis. At that place are invasive and not-invasive methods of measuring PP.[ citation needed ]
Finapres methodology [edit]
In 1967, the Czech physiologist Jan Peňáz invented and patented the volume clamp method of measuring continuous claret pressure. The principle of the volume clamp method is to dynamically provide equal pressures, on either side of an artery wall. By clamping the artery to a certain volume, inside pressure level—intra-arterial pressure level—balances exterior pressure—finger cuff pressure level. Peñáz decided the finger was the optimal site to apply this volume clamp method. The use of finger cuffs excludes the device from application in patients without vasoconstriction, such as in sepsis or in patients on vasopressors.[ commendation needed ]
In 1978, scientists at BMI-TNO, the research unit of Netherlands System for Applied Scientific Research at the University of Amsterdam, invented and patented a serial of additional key elements that make the volume clamp piece of work in clinical exercise. These methods include the apply of modulated infrared light in the optical system inside the sensor, the lightweight, easy-to-wrap finger cuff with velcro fixation, a new pneumatic proportional command valve principle, and a set point strategy for the determining and tracking the correct volume at which to clench the finger arteries—the Physiocal system. An acronym for physiological calibration of the finger arteries, this Physiocal tracker was found to be accurate, robust and reliable.[ citation needed ]
The Finapres methodology was developed to use this information to calculate arterial pressure from finger cuff pressure data. A generalised algorithm to correct for the pressure deviation between the finger and brachial sites in patients was developed. This correction worked nether all of the circumstances information technology was tested in—even when information technology was not designed for it—considering it applied general physiological principles. This innovative brachial pressure level waveform reconstruction method was first implemented in the Finometer, the successor of Finapres that BMI-TNO introduced to the market place in 2000.[ citation needed ]
The availability of a continuous, high-fidelity, calibrated blood pressure level waveform opened up the perspective of crush-to-beat computation of integrated haemodynamics, based on 2 notions: pressure and period are inter-related at each site in the arterial organisation by their so-chosen characteristic impedance. At the proximal aortic site, the iii-chemical element Windkessel model of this impedance can be modelled with sufficient accurateness in an individual patient with known age, gender, top and weight. According to comparisons of non-invasive peripheral vascular monitors, modest clinical utility is restricted to patients with normal and invariant circulation.[27]
Invasive [edit]
Invasive PP monitoring involves inserting a manometer pressure level sensor into an artery—ordinarily the radial or femoral artery—and continuously measuring the PP waveform. This is more often than not done past connecting the catheter to a signal processing device with a display. The PP waveform tin so be analysed to provide measurements of cardiovascular performance. Changes in vascular part, the position of the catheter tip or damping of the pressure level waveform signal will bear upon the accuracy of the readings. Invasive PP measurements can be calibrated or uncalibrated.[ commendation needed ]
Calibrated PP – PiCCO, LiDCO [edit]
PiCCO (PULSION Medical Systems AG, Munich, Germany) and PulseCO (LiDCO Ltd, London, England) generate continuous Q by analysing the arterial PP waveform. In both cases, an independent technique is required to provide scale of continuous Q analysis because arterial PP analysis cannot business relationship for unmeasured variables such as the changing compliance of the vascular bed. Recalibration is recommended later changes in patient position, therapy or status.[ commendation needed ]
In PiCCO, transpulmonary thermodilution, which uses the Stewart-Hamilton principle but measures temperatures changes from key venous line to a fundamental arterial line, i.e., the femoral or axillary arterial line, is used as the calibrating technique. The Q value derived from cold-saline thermodilution is used to calibrate the arterial PP contour, which can then provide continuous Q monitoring. The PiCCO algorithm is dependent on blood pressure waveform morphology (mathematical analysis of the PP waveform), and it calculates continuous Q equally described by Wesseling and colleagues.[28] Transpulmonary thermodilution spans right eye, pulmonary circulation and left heart, allowing further mathematical assay of the thermodilution curve and giving measurements of cardiac filling volumes (GEDV), intrathoracic claret volume and extravascular lung h2o. Transpulmonary thermodilution allows for less invasive Q calibration simply is less accurate than PA thermodilution and requires a central venous and arterial line with the accompanied infection risks.[ commendation needed ]
In LiDCO, the independent scale technique is lithium chloride dilution using the Stewart-Hamilton principle. Lithium chloride dilution uses a peripheral vein and a peripheral arterial line. Like PiCCO, frequent scale is recommended when at that place is a change in Q.[29] Calibration events are limited in frequency because they involve the injection of lithium chloride and can exist subject field to errors in the presence of certain muscle relaxants. The PulseCO algorithm used past LiDCO is based on pulse power derivation and is not dependent on waveform morphology.[ citation needed ]
Statistical analysis of arterial pressure – FloTrac/Vigileo [edit]

FloTrac/Vigileo (Edwards Lifesciences) is an uncalibrated, haemodynamic monitor based on pulse contour analysis. It estimates cardiac output (Q) using a standard arterial catheter with a manometer located in the femoral or radial artery. The device consists of a high-fidelity pressure transducer, which, when used with a supporting monitor (Vigileo or EV1000 monitor), derives left-sided cardiac output (Q) from a sample of arterial pulsations. The device uses an algorithm based on the Frank–Starling police of the heart, which states pulse pressure (PP) is proportional to stroke volume (SV). The algorithm calculates the product of the standard deviation of the arterial pressure (AP) wave over a sampled period of 20 seconds and a vascular tone factor (Khi, or χ) to generate stroke volume. The equation in simplified form is: , or, . Khi is designed to reflect arterial resistance; compliance is a multivariate polynomial equation that continuously quantifies arterial compliance and vascular resistance. Khi does this by analyzing the morphological changes of arterial force per unit area waveforms on a bit-past-bit ground, based on the principle that changes in compliance or resistance touch on the shape of the arterial force per unit area waveform. By analyzing the shape of said waveforms, the effect of vascular tone is assessed, assuasive the calculation of SV. Q is then derived using equation (i). But perfused beats that generate an arterial waveform are counted for in Hr.[ commendation needed ]
This arrangement estimates Q using an existing arterial catheter with variable accuracy. These arterial monitors exercise non crave intracardiac catheterisation from a pulmonary artery catheter. They crave an arterial line and are therefore invasive. As with other arterial waveform systems, the short set-up and data acquisition times are benefits of this technology. Disadvantages include its inability to provide data regarding correct-sided center pressures or mixed venous oxygen saturation.[thirty] [31] The measurement of Stroke Book Variation (SVV), which predicts book responsiveness is intrinsic to all arterial waveform technologies. Information technology is used for managing fluid optimisation in high-risk surgical or critically ill patients. A physiologic optimization program based on haemodynamic principles that incorporates the data pairs SV and SVV has been published.[32]
Arterial monitoring systems are unable to predict changes in vascular tone; they estimate changes in vascular compliance. The measurement of force per unit area in the artery to calculate the flow in the heart is physiologically irrational and of questionable accurateness,[33] and of unproven benefit.[34] Arterial pressure monitoring is limited in patients off-ventilation, in atrial fibrillation, in patients on vasopressors, and in those with a dynamic autonomic system such as those with sepsis.[29]
Uncalibrated, pre-estimated demographic data-free – PRAM [edit]
Pressure Recording Analytical Method (PRAM), estimates Q from the analysis of the pressure moving ridge profile obtained from an arterial catheter—radial or femoral access. This PP waveform tin can so exist used to decide Q. Equally the waveform is sampled at 1000 Hz, the detected pressure curve tin be measured to calculate the actual beat-to-vanquish stroke volume. Unlike FloTrac, neither constant values of impedance from external calibration, nor form pre-estimated in vivo or in vitro information, are needed.
PRAM has been validated against the considered gold standard methods in stable condition[35] and in diverse haemodynamic states.[36] It tin exist used to monitor pediatric and mechanically supported patients.[37] [38]
Generally monitored haemodynamic values, fluid responsiveness parameters and an exclusive reference are provided past PRAM: Cardiac Cycle Efficiency (CCE). It is expressed past a pure number ranging from one (all-time) to -1 (worst) and information technology indicates the overall eye-vascular response coupling. The ratio between center performance and consumed free energy, represented every bit CCE "stress alphabetize", can exist of paramount importance in understanding the patient's present and future courses.[39]
Impedance cardiography [edit]
Impedance cardiography (often abbreviated equally ICG, or Thoracic Electrical Bioimpedance (TEB)) measures changes in electrical impedance across the thoracic region over the cardiac cycle. Lower impedance indicates greater intrathoracic fluid volume and blood flow. By synchronizing fluid volume changes with the heartbeat, the alter in impedance can be used to calculate stroke volume, cardiac output and systemic vascular resistance.[40]
Both invasive and not-invasive approaches are used.[41] The reliability and validity of the not-invasive approach has gained some acceptance,[42] [43] [44] [45] although in that location is non complete agreement on this point.[46] The clinical utilise of this approach in the diagnosis, prognosis and therapy of a diverseness of diseases continues.[47]
Not-invasive ICG equipment includes the Bio-Z Dx,[48] the Niccomo,[49] and TEBCO products by BoMed.[50] [51]
Ultrasound dilution [edit]
Ultrasound dilution (UD) uses body-temperature normal saline (NS) as an indicator introduced into an extracorporeal loop to create an atriovetricular (AV) circulation with an ultrasound sensor, which is used to measure the dilution then to calculate cardiac output using a proprietary algorithm. A number of other haemodynamic variables, such equally total end-diastole volume (TEDV), primal blood volume (CBV) and active circulation volume (ACVI) can be calculated using this method.[ citation needed ]
The UD method was firstly introduced in 1995.[52] It was extensively used to measure out catamenia and volumes with extracorporeal circuit conditions, such as ECMO[53] [54] and Haemodialysis,[55] [56] leading more than than 150 peer reviewed publications. UD has now been adapted to intensive care units (ICU) as the COstatus device.[57]
The UD method is based on ultrasound indicator dilution.[58] Blood ultrasound velocity (1560–1585 1000/s) is a function of full blood protein concentration—sums of proteins in plasma and in red claret red cells—and temperature. Injection of body-temperature normal saline (ultrasound velocity of saline is 1533 m/s) into a unique AV loop decreases blood ultrasound velocity, and produces dilution curves.[ citation needed ]
UD requires the establishment of an extracorporeal apportionment through its unique AV loop with two pre-existing arterial and central venous lines in ICU patients. When the saline indicator is injected into the AV loop, it is detected by the venous clench-on sensor on the loop before it enters the patient's centre's right atrium. Later the indicator traverses the centre and lung, the concentration curve in the arterial line is recorded and displayed on the COstatus HCM101 Monitor. Cardiac output is calculated from the expanse of the concentration curve using the Stewart-Hamilton equation. UD is a non-invasive process, requiring only a connection to the AV loop and two lines from a patient. UD has been specialised for application in pediatric ICU patients and has been demonstrated to be relatively safety although invasive and reproducible.[ citation needed ]
Electrical cardiometry [edit]

Electrode array that measures Thoracic electrical bioimpedance (TEB)
Electrical cardiometry is a non-invasive method like to Impedance cardiography; both methods measure thoracic electrical bioimpedance (TEB). The underlying model differs between the two methods; Electrical cardiometry attributes the steep increment of TEB beat-to-beat to the change in orientation of crimson blood cells. Iv standard ECG electrodes are required for measurement of cardiac output. Electrical Cardiometry is a method trademarked past Cardiotronic, Inc., and shows promising results in a wide range of patients. It is currently approved in the US for employ in adults, children and babies. Electrical cardiometry monitors have shown promise in postoperative cardiac surgical patients, in both haemodynamicially stable and unstable cases.[59]
Magnetic resonance imaging [edit]
Velocity-encoded phase contrast Magnetic resonance imaging (MRI)[sixty] is the nearly authentic technique for measuring menstruum in large vessels in mammals. MRI period measurements have been shown to exist highly accurate compared to measurements fabricated with a chalice and timer,[61] and less variable than the Fick principle[62] and thermodilution.[63]
Velocity-encoded MRI is based on the detection of changes in the phase of proton precession. These changes are proportional to the velocity of the protons' movement through a magnetic field with a known gradient. When using velocity-encoded MRI, the result is two sets of images, one for each time point in the cardiac wheel. 1 is an anatomical image and the other is an image in which the signal intensity in each pixel is straight proportional to the through-airplane velocity. The average velocity in a vessel, i.e., the aorta or the pulmonary artery, is quantified by measuring the average bespeak intensity of the pixels in the cantankerous-department of the vessel then multiplying past a known constant. The flow is calculated by multiplying the hateful velocity by the cross-sectional surface area of the vessel. This flow information can be used in a menstruum-versus-time graph. The area under the flow-versus-fourth dimension curve for one cardiac bike is the stroke volume. The length of the cardiac cycle is known and determines heart rate; Q tin be calculated using equation (i). MRI is typically used to quantify the flow over one cardiac cycle every bit the average of several heart beats. It is also possible to quantify the stroke volume in existent-time on a beat-for-beat ground.[64]
While MRI is an important research tool for accurately measuring Q, it is currently non clinically used for haemodynamic monitoring in emergency or intensive care settings. As of 2015[update], cardiac output measurement by MRI is routinely used in clinical cardiac MRI examinations.[65]
Dye dilution method [edit]
The dye dilution method is done by rapidly injecting a dye, indocyanine green, into the right atrium of the heart. The dye flows with the blood into the aorta. A probe is inserted into the aorta to mensurate the concentration of the dye leaving the heart at equal time intervals [0, T] until the dye has cleared. Permit c(t) be the concentration of the dye at time t. By dividing the fourth dimension intervals from [0, T] into subintervals Δt, the amount of dye that flows past the measuring point during the subinterval from to is:
where is the charge per unit of flow that is beingness calculated. The total corporeality of dye is:
and, letting , the amount of dye is:
Thus, the cardiac output is given by:
where the corporeality of dye injected is known, and the integral can be determined using the concentration readings.[66]
The dye dilution method is 1 of the most authentic methods of determining cardiac output during exercise. The error of a single calculation of cardiac output values at rest and during practice is less than 5%. This method does not allow measurement of 'beat to beat' changes, and requires a cardiac output that is stable for approximately x southward during exercise and 30 south at residuum.[ citation needed ]
Factors influencing cardiac output [edit]

Hierarchical summary of major factors influencing cardiac output.
Cardiac output is primarily controlled past the oxygen requirement of tissues in the trunk. In contrast to other pump systems, the eye is a demand pump that does not regulate its own output.[67] When the body has a high metabolic oxygen demand, the metabolically controlled flow through the tissues is increased, leading to a greater menses of blood back to the center, leading to higher cardiac output.
The capacitance, also known as compliance, of the arterio-vascular channels that carry the claret also controls cardiac output. As the torso's blood vessels actively expand and contract, the resistance to blood menstruation decreases and increases respectively. Thin-walled veins have about eighteen times the capacitance of thick-walled arteries because they are able to carry more blood by virtue of being more than distensible.[68]
From this formula, it is clear the factors affecting stroke volume and heart rate as well affect cardiac output. The figure to the right illustrates this dependency and lists a few of these factors. A more than detailed hierarchical illustration is provided in a subsequent effigy.
Equation (1) reveals Hr and SV to be the master determinants of cardiac output Q. A detailed representation of these factors is illustrated in the effigy to the right. The primary factors that influence HR are autonomic innervation plus endocrine control. Environmental factors, such equally electrolytes, metabolic products, and temperature are not shown. The determinants of SV during the cardiac cycle are the contractility of the heart musculus, the degree of preload of myocardial distention prior to shortening and the afterload during ejection.[69] Other factors such every bit electrolytes may be classified as either positive or negative inotropic agents.[70]
Cardiac response [edit]
| |||||||||||||||
|
Clinical significance [edit]
When Q increases in a healthy simply untrained individual, most of the increase tin exist attributed to an increase in heart rate (HR). Change of posture, increased sympathetic nervous system activity, and decreased parasympathetic nervous system activity can besides increase cardiac output. Hr can vary by a gene of approximately 3—between 60 and 180 beats per infinitesimal—while stroke book (SV) can vary between 70 and 120 ml (ii.5 and iv.2 imp fl oz; 2.4 and 4.1 Usa fl oz), a cistron of only one.seven.[71] [72] [73]
Diseases of the cardiovascular organisation are frequently associated with changes in Q, particularly the pandemic diseases hypertension and heart failure. Increased Q tin exist associated with cardiovascular disease that can occur during infection and sepsis. Decreased Q can exist associated with cardiomyopathy and heart failure.[69] Sometimes, in the presence of ventricular illness associated with dilatation, EDV may vary. An increment in EDV could counterbalance LV dilatation and dumb contraction. From equation (3), the resulting cardiac output Q may remain constant. The ability to accurately mensurate Q is of import in clinical medicine because information technology provides for improved diagnosis of abnormalities and can exist used to guide advisable management.[74]
Example values [edit]
| Ventricular volumes | ||
|---|---|---|
| Measure | Correct ventricle | Left ventricle |
| End-diastolic volume | 144 mL(± 23 mL)[75] | 142 mL (± 21 mL)[76] |
| End-diastolic volume / trunk expanse (mL/chiliadii) | 78 mL/m2 (± 11 mL/thousand2)[75] | 78 mL/yard2 (± 8.8 mL/thou2)[76] |
| End-systolic volume | l mL (± 14 mL)[75] | 47 mL (± x mL)[76] |
| Stop-systolic volume / body surface area (mL/mtwo) | 27 mL/thousand2 (± 7 mL/mtwo)[75] | 26 mL/m2 (± five.ane mL/yardii)[76] |
| Stroke volume | 94 mL (± 15 mL)[75] | 95 mL (± fourteen mL)[76] |
| Stroke volume / trunk expanse (mL/mii) | 51 mL/k2 (± 7 mL/m2)[75] | 52 mL/thou2 (± half-dozen.2 mL/10002)[76] |
| Ejection fraction | 66% (± half-dozen%)[75] | 67% (± four.6%)[76] |
| Centre rate | sixty–100 bpm[77] | 60–100 bpm[77] |
| Cardiac output | 4.0–8.0 L/minute[78] | 4.0–eight.0 l L/infinitesimal[78] |
[edit]
Ejection fraction [edit]
Ejection fraction (EF) is a parameter related to SV. EF is the fraction of blood ejected by the left ventricle (LV) during the contraction or ejection phase of the cardiac bicycle or systole. Prior to the beginning of systole, during the filling phase or diastole, the LV is filled with claret to the capacity known equally end diastolic book (EDV). During systole, the LV contracts and ejects claret until information technology reaches its minimum capacity known every bit end systolic book (ESV). It does not completely empty. The following equations assist translate the upshot of EF and EDV on cardiac output Q, via SV.
-
-
(3)
-
Cardiac input [edit]
Cardiac input (CI) is the inverse operation of cardiac output. Every bit cardiac output implies the volumetric expression of ejection fraction, cardiac input implies the volumetric injection fraction (IF).
IF = end diastolic book (EDV) / terminate systolic book (ESV)
Cardiac input is a readily imaged mathematical model of diastole. [ clarification needed ]
Cardiac alphabetize [edit]
In all resting mammals of normal mass, CO value is a linear function of body mass with a slope of 0.one 50/(min kg).[79] [80] Fatty has most 65% of oxygen demand per mass in comparison to other lean torso tissues. Equally a result, the calculation of normal CO value in an obese subject is more complex; a single, mutual "normal" value of SV and CO for adults cannot exist. All blood flow parameters take to be indexed. It is accepted convention to index them by the Trunk Surface area, BSA [m2], by DuBois & DuBois Formula, a function of height and weight:
The resulting indexed parameters are Stroke Index (SI) and Cardiac Index (CI). Stroke Index, measured in ml/beat/grandtwo, is divers equally
Cardiac Alphabetize, measured in L/min/m2, is defined as
The CO equation (one) for indexed parameters so changes to the following.
-
(2)
The normal range for these indexed blood flow parameters are betwixt 35 and 65 mL/beat/m2 for SI and between 2.5 and 4 Fifty/(min chiliad2) for CI.[81]
Combined cardiac output [edit]
Combined cardiac output (CCO) is the sum of the outputs of the correct and left sides of the center. It is a useful measurement in fetal circulation, where cardiac outputs from both sides of the center work partly in parallel by the foramen ovale and ductus arteriosus, which directly supply the systemic apportionment.[82]
Historical methods [edit]
Fick principle [edit]

An analogy of how spirometry is done
The Fick principle, showtime described by Adolf Eugen Fick in 1870, assumes the charge per unit of oxygen consumption is a office of the rate of blood flow and the charge per unit of oxygen picked upwardly by the ruddy blood cells. Awarding of the Fick principle involves calculating the oxygen consumed over time by measuring the oxygen concentration of venous blood and arterial blood. Q is calculated from these measurements as follows:
- 5 O2 consumption per minute using a spirometer (with the subject area re-breathing air) and a CO2 absorber
- the oxygen content of blood taken from the pulmonary avenue (representing mixed venous blood)
- the oxygen content of blood from a cannula in a peripheral avenue (representing arterial claret)
From these values, nosotros know that:
where
- C A is the oxygen content of arterial blood, and,
- C Five is the oxygen content of venous blood.
This allows u.s. to say
and therefore summate Q. (CA – CFive ) is too known equally the arteriovenous oxygen difference.[83]
While considered to be the well-nigh accurate method of measuring Q, the Fick method is invasive and requires time for sample analysis, and accurate oxygen consumption samples are hard to acquire. There have been modifications to the Fick method where respiratory oxygen content is measured as part of a closed system and the consumed oxygen is calculated using an assumed oxygen consumption index, which is then used to calculate Q. Other variations use inert gases as tracers and measure the alter in inspired and expired gas concentrations to summate Q (Innocor, Innovision A/Due south, Kingdom of denmark).
The calculation of the arterial and venous oxygen content of the blood is a straightforward process. Almost all oxygen in the blood is leap to hæmoglobin molecules in the crimson blood cells. Measuring the content of hæmoglobin in the blood and the percent of saturation of hæmoglobin—the oxygen saturation of the blood—is a simple process and is readily available to physicians. Each gram of haemoglobin can bear 1.34 mL of Oii; the oxygen content of the blood—either arterial or venous—tin be estimated using the following formula:
Pulmonary artery thermodilution (trans-right-eye thermodilution) [edit]

Diagram of Pulmonary avenue catheter (PAC)
The indicator method was further developed by replacing the indicator dye with heated or cooled fluid. Temperature changes rather than dye concentration are measured at sites in the circulation; this method is known as thermodilution. The pulmonary artery catheter (PAC) introduced to clinical practice in 1970, too known as the Swan-Ganz catheter, provides directly access to the right center for thermodilution measurements. Continuous, invasive, cardiac monitoring in intensive care units has been mostly phased out. The PAC remains useful in correct-heart report washed in cardiac catheterisation laboratories.[ citation needed ]
The PAC is airship tipped and is inflated, which helps "canvas" the catheter airship through the right ventricle to occlude a small-scale branch of the pulmonary artery system. The airship is then deflated. The PAC thermodilution method involves the injection of a small corporeality (10 mL) of common cold glucose at a known temperature into the pulmonary artery and measuring the temperature a known altitude abroad 6–x cm (ii.4–three.9 in) using the same catheter with temperature sensors gear up apart at a known distance.[ citation needed ]
The historically significant Swan-Ganz multi-lumen catheter allows reproducible adding of cardiac output from a measured fourth dimension-temperature curve, besides known equally the thermodilution curve. Thermistor technology enabled the observations that low CO registers temperature modify slowly and high CO registers temperature change rapidly. The caste of temperature change is direct proportional to the cardiac output. In this unique method, three or four repeated measurements or passes are usually averaged to improve accuracy.[84] [85] Modernistic catheters are fitted with heating filaments that intermittently rut up and measure the thermodilution curve, providing serial Q measurements. These instruments average measurements over ii–9 minutes depending on the stability of the circulation, and thus do non provide continuous monitoring.
PAC use can be complicated past arrhythmias, infection, pulmonary avenue rupture and damage to the right middle valve. Recent studies in patients with critical illnesses, sepsis, acute respiratory failure and centre failure propose that use of the PAC does not better patient outcomes.[5] [half-dozen] [7] This clinical ineffectiveness may relate to its poor accuracy and sensitivity, which have been demonstrated by comparing with catamenia probes across a sixfold range of Q values.[13] Employ of PAC is in pass up equally clinicians move to less invasive and more accurate technologies for monitoring hæmodynamics.[86]
References [edit]
- ^ a b c d due east f g h i j m l thou due north o p q r due south Betts JG (2013). Anatomy & physiology. pp. 787–846. ISBN978-1938168130 . Retrieved 11 August 2014.
- ^ Kenyon, Anna; Williams, David; Adamson, Dawn (x June 2010). "Physiology". Basic Science in Obstetrics and Gynaecology. Elsevier. pp. 173–230. doi:10.1016/b978-0-443-10281-3.00014-ii. ISBN978-0-443-10281-3. OCLC 1023146175. edited by Catherine Eastward. Williamson, Phillip Bennett
- ^ OpenStax (vi March 2013). "Cardiac Physiology". BC Open Textbooks – Open Textbooks Adjusted and Created by BC Faculty. Archived from the original on vi November 2021. Retrieved 7 April 2020.
- ^ Dunn, J.-Oc; Mythen, M. Thou.; Grocott, M. P. (1 October 2016). "Physiology of Oxygen Transport". BJA Education. xvi (ten): 341–48. doi:x.1093/bjaed/mkw012. ISSN 2058-5349. Archived from the original on 23 February 2022.
- ^ a b Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko Thou, Stevenson LW, Francis GS, Leier CV, Miller LW (Oct 2005). "Evaluation report of congestive heart failure and pulmonary avenue catheterization effectiveness: the ESCAPE trial". JAMA. 294 (13): 1625–33. doi:10.1001/jama.294.13.1625. PMID 16204662.
- ^ a b Pasche B, Knobloch TJ, Bian Y, Liu J, Phukan South, Rosman D, Kaklamani V, Baddi 50, Siddiqui FS, Frankel W, Prior TW, Schuller DE, Agrawal A, Lang J, Dolan ME, Vokes EE, Lane WS, Huang CC, Caldes T, Di Cristofano A, Hampel H, Nilsson I, von Heijne M, Fodde R, Murty VV, de la Chapelle A, Weghorst CM (October 2005). "Somatic acquisition and signaling of TGFBR1*6A in cancer". JAMA. 294 (thirteen): 1634–46. doi:10.1001/jama.294.13.1634. PMID 16204663.
- ^ a b Hall JB (October 2005). "Searching for evidence to support pulmonary artery catheter use in critically sick patients". JAMA. 294 (13): 1693–94. doi:10.1001/jama.294.13.1693. PMID 16204671.
- ^ Finegold JA, Manisty CH, Cecaro F, Sutaria Due north, Mayet J, Francis DP (August 2013). "Choosing betwixt velocity-time-integral ratio and peak velocity ratio for adding of the dimensionless index (or aortic valve surface area) in serial follow-upward of aortic stenosis". International Journal of Cardiology. 167 (iv): 1524–31. doi:10.1016/j.ijcard.2012.04.105. PMID 22575631.
- ^ Su BC, Yu HP, Yang MW, Lin CC, Kao MC, Chang CH, Lee WC (July 2008). "Reliability of a new ultrasonic cardiac output monitor in recipients of living donor liver transplantation". Liver Transplantation. 14 (7): 1029–37. doi:ten.1002/lt.21461. PMID 18581505. S2CID 37185399.
- ^ Phillips R, Paradisis Chiliad, Evans North, Southwell D, Burstow D, West M (2006). "Cardiac output measurement in preterm neonates: validation of USCOM against echocardiography". Critical Intendance. 10 (Suppl ane): P343. doi:10.1186/cc4690. PMC4092718.
- ^ Cattermole GN, Leung PY, Mak PS, Chan SS, Graham CA, Rainer TH (September 2010). "The normal ranges of cardiovascular parameters in children measured using the Ultrasonic Cardiac Output Monitor". Critical Care Medicine. 38 (9): 1875–81. doi:10.1097/CCM.0b013e3181e8adee. PMID 20562697. S2CID 24949904.
- ^ Jain Due south, Allins A, Salim A, Vafa A, Wilson MT, Margulies DR (December 2008). "Noninvasive Doppler ultrasonography for assessing cardiac function: tin it replace the Swan-Ganz catheter?". American Journal of Surgery. 196 (6): 961–67, discussion 967–68. doi:10.1016/j.amjsurg.2008.07.039. PMID 19095116.
- ^ a b Phillips RA, Hood SG, Jacobson BM, West MJ, Wan L, May CN (2012). "Pulmonary Artery Catheter (PAC) Accuracy and Efficacy Compared with Menses Probe and Transcutaneous Doppler (USCOM): An Ovine Cardiac Output Validation". Critical Care Inquiry and Practise. 2012: 1–9. doi:10.1155/2012/621496. PMC3357512. PMID 22649718.
- ^ Horster S, Stemmler HJ, Strecker N, Brettner F, Hausmann A, Cnossen J, Parhofer KG, Nickel T, Geiger South (2012). "Cardiac Output Measurements in Septic Patients: Comparison the Accuracy of USCOM to PiCCO". Critical Care Inquiry and Do. 2012: one–5. doi:x.1155/2012/270631. PMC3235433. PMID 22191019.
- ^ Phillips R, Lichtenthal P, Sloniger J, Burstow D, Westward M, Copeland J (March 2009). "Noninvasive cardiac output measurement in center failure subjects on circulatory support". Anesthesia and Analgesia. 108 (iii): 881–86. doi:10.1213/one.0b013e318193174b. PMID 19224797. S2CID 35618846.
- ^ Kager CC, Dekker GA, Stam MC (April 2009). "Measurement of cardiac output in normal pregnancy by a non-invasive ii-dimensional independent Doppler device". The Australian & New Zealand Journal of Obstetrics & Gynaecology. 49 (2): 142–44. doi:10.1111/j.1479-828X.2009.00948.x. PMID 19441163. S2CID 25371483.
- ^ Mythen MG, Webb AR (April 1995). "Perioperative plasma book expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery". Archives of Surgery. 130 (4): 423–29. doi:10.1001/archsurg.1995.01430040085019. PMID 7535996.
- ^ Sinclair S, James South, Singer M (October 1997). "Intraoperative intravascular book optimisation and length of hospital stay afterward repair of proximal femoral fracture: randomised controlled trial". BMJ. 315 (7113): 909–12. doi:10.1136/bmj.315.7113.909. PMC2127619. PMID 9361539.
- ^ Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C (September 2002). "Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery". Anaesthesia. 57 (9): 845–49. doi:ten.1046/j.1365-2044.2002.02708.x. PMID 12190747. S2CID 43755776.
- ^ Gan TJ, Soppitt A, Maroof Thou, el-Moalem H, Robertson KM, Moretti Eastward, Dwane P, Glass PS (October 2002). "Goal-directed intraoperative fluid administration reduces length of infirmary stay afterwards major surgery". Anesthesiology. 97 (4): 820–26. doi:10.1097/00000542-200210000-00012. PMID 12357146. S2CID 10471164.
- ^ Venn R, Steele A, Richardson P, Poloniecki J, Grounds Thousand, Newman P (January 2002). "Randomized controlled trial to investigate influence of the fluid challenge on duration of infirmary stay and perioperative morbidity in patients with hip fractures". British Journal of Anaesthesia. 88 (1): 65–71. doi:x.1093/bja/88.ane.65. PMID 11881887.
- ^ Wakeling HG, McFall MR, Jenkins CS, Forest WG, Miles WF, Barclay GR, Fleming SC (November 2005). "Intraoperative oesophageal Doppler guided fluid direction shortens postoperative hospital stay later on major bowel surgery". British Periodical of Anaesthesia. 95 (5): 634–42. doi:x.1093/bja/aei223. PMID 16155038.
- ^ Noblett SE, Snowden CP, Shenton BK, Horgan AF (September 2006). "Randomized clinical trial assessing the consequence of Doppler-optimized fluid management on event after elective colorectal resection". The British Journal of Surgery. 93 (nine): 1069–76. doi:10.1002/bjs.5454. PMID 16888706. S2CID 25469534.
- ^ Pillai P, McEleavy I, Gaughan Chiliad, Snowden C, Nesbitt I, Durkan G, Johnson Chiliad, Cosgrove J, Thorpe A (December 2011). "A double-blind randomized controlled clinical trial to assess the issue of Doppler optimized intraoperative fluid management on outcome following radical cystectomy". The Periodical of Urology. 186 (vi): 2201–06. doi:10.1016/j.juro.2011.07.093. PMID 22014804.
- ^ "CardioQ-ODM oesophageal doppler monitor | Guidance | NICE".
- ^ Lowe GD, Chamberlain BM, Philpot EJ, Willshire RJ (2010). "Oesophageal Doppler Monitor (ODM) guided individualised goal directed fluid management (iGDFM) in surgery – a technical review" (PDF). Deltex Medical Technical Review. Archived from the original (PDF) on 23 September 2015. Retrieved 13 October 2014.
- ^ de Wilde RB, Schreuder JJ, van den Berg PC, Jansen JR (August 2007). "An evaluation of cardiac output past five arterial pulse contour techniques during cardiac surgery". Anaesthesia. 62 (eight): 760–68. doi:10.1111/j.1365-2044.2007.05135.x. PMID 17635422.
- ^ Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ (May 1993). "Computation of aortic menstruation from force per unit area in humans using a nonlinear, 3-element model". Journal of Practical Physiology. 74 (five): 2566–73. doi:10.1152/jappl.1993.74.5.2566. PMID 8335593.
- ^ a b Bein B, Meybohm P, Cavus E, Renner J, Tonner PH, Steinfath M, Scholz J, Doerges V (July 2007). "The reliability of pulse contour-derived cardiac output during hemorrhage and after vasopressor administration". Anesthesia and Analgesia. 105 (1): 107–13. doi:10.1213/01.ane.0000268140.02147.ed. PMID 17578965. S2CID 5549744.
- ^ Singh S, Taylor MA (August 2010). "Con: the FloTrac device should not exist used to follow cardiac output in cardiac surgical patients". Periodical of Cardiothoracic and Vascular Anesthesia. 24 (4): 709–11. doi:x.1053/j.jvca.2010.04.023. PMID 20673749.
- ^ Manecke GR (September 2005). "Edwards FloTrac sensor and Vigileo monitor: easy, authentic, reliable cardiac output assessment using the arterial pulse moving ridge". Skillful Review of Medical Devices. 2 (v): 523–27. doi:10.1586/17434440.two.five.523. PMID 16293062. S2CID 31049402.
- ^ McGee WT (2009). "A elementary physiologic algorithm for managing hemodynamics using stroke volume and stroke volume variation: physiologic optimization programme". Journal of Intensive Care Medicine. 24 (6): 352–threescore. doi:ten.1177/0885066609344908. PMID 19736180. S2CID 12806349.
- ^ Su BC, Tsai YF, Chen CY, Yu HP, Yang MW, Lee WC, Lin CC (March 2012). "Cardiac output derived from arterial pressure waveform assay in patients undergoing liver transplantation: validity of a 3rd-generation device". Transplantation Proceedings. 44 (2): 424–28. doi:ten.1016/j.transproceed.2011.12.036. PMID 22410034.
- ^ Takala J, Ruokonen E, Tenhunen JJ, Parviainen I, Jakob SM (June 2011). "Early non-invasive cardiac output monitoring in hemodynamically unstable intensive care patients: a multi-center randomized controlled trial". Critical Care. xv (3): R148. doi:10.1186/cc10273. PMC3219022. PMID 21676229.
- ^ Romano SM, Pistolesi One thousand (Baronial 2002). "Assessment of cardiac output from systemic arterial pressure in humans". Disquisitional Intendance Medicine. 30 (8): 1834–41. doi:10.1097/00003246-200208000-00027. PMID 12163802. S2CID 12100251.
- ^ Scolletta S, Romano SM, Biagioli B, Capannini G, Giomarelli P (August 2005). "Pressure recording belittling method (PRAM) for measurement of cardiac output during various haemodynamic states". British Periodical of Anaesthesia. 95 (2): 159–65. doi:10.1093/bja/aei154. PMID 15894561.
- ^ Calamandrei Thousand, Mirabile L, Muschetta Southward, Gensini GF, De Simone L, Romano SM (May 2008). "Cess of cardiac output in children: a comparing between the pressure recording analytical method and Doppler echocardiography". Pediatric Disquisitional Care Medicine. 9 (3): 310–12. doi:10.1097/PCC.0b013e31816c7151. PMID 18446106. S2CID 25815656.
- ^ Scolletta S, Gregoric ID, Muzzi L, Radovancevic B, Frazier OH (January 2007). "Pulse moving ridge analysis to assess systemic blood flow during mechanical biventricular support". Perfusion. 22 (1): 63–66. doi:x.1177/0267659106074784. PMID 17633137. S2CID 32129645.
- ^ Scolletta Due south, Romano SM, Maglioni H (2005). "Left ventricular performance by PRAM during cardiac surgery". p. S157. in "OP 564–605". Intensive Care Medicine. 31 (Suppl 1): S148–58. 2005. doi:10.1007/s00134-005-2781-iii. S2CID 30752685.
- ^ Bernstein, Donald P (2010). "Impedance cardiography: Pulsatile blood flow and the biophysical and electrodynamic footing for the stroke book equations". Journal of Electrical Bioimpedance. 1: ii–17. doi:10.5617/jeb.51. Archived from the original on 17 Oct 2015.
- ^ Costa PD, Rodrigues PP, Reis AH, Costa-Pereira A (December 2010). "A review on remote monitoring engineering science applied to implantable electronic cardiovascular devices". Telemedicine Periodical and East-Health. 16 (ten): 1042–50. doi:ten.1089/tmj.2010.0082. PMID 21070132.
- ^ Tang WH, Tong W (March 2009). "Measuring impedance in congestive eye failure: electric current options and clinical applications". American Centre Periodical. 157 (3): 402–11. doi:10.1016/j.ahj.2008.x.016. PMC3058607. PMID 19249408.
- ^ Ferrario CM, Flack JM, Strobeck JE, Smits Thou, Peters C (February 2010). "Individualizing hypertension treatment with impedance cardiography: a meta-analysis of published trials". Therapeutic Advances in Cardiovascular Affliction. iv (1): v–16. doi:x.1177/1753944709348236. PMID 20042450.
- ^ Moshkovitz Y, Kaluski Due east, Milo O, Vered Z, Cotter Thou (May 2004). "Contempo developments in cardiac output conclusion by bioimpedance: comparison with invasive cardiac output and potential cardiovascular applications". Electric current Opinion in Cardiology. 19 (3): 229–37. doi:10.1097/00001573-200405000-00008. PMID 15096956. S2CID 28996732.
- ^ Parry MJ, McFetridge-Durdle J (2006). "Convalescent impedance cardiography: a systematic review". Nursing Inquiry. 55 (4): 283–91. doi:ten.1097/00006199-200607000-00009. PMID 16849981. S2CID 28726590.
- ^ Wang DJ, Gottlieb SS (September 2006). "Impedance cardiography: more questions than answers". Electric current Heart Failure Reports. 3 (3): 107–xiii. doi:ten.1007/s11897-006-0009-7. PMID 16914102. S2CID 31094943.
- ^ Ventura HO, Taler SJ, Strobeck JE (Feb 2005). "Hypertension as a hemodynamic disease: the role of impedance cardiography in diagnostic, prognostic, and therapeutic decision making". American Periodical of Hypertension. 18 (2 Pt two): 26S–43S. doi:ten.1016/j.amjhyper.2004.11.002. PMID 15752931.
- ^ "Archived re-create". Archived from the original on 3 December 2010. Retrieved thirty November 2010.
{{cite spider web}}: CS1 maint: archived copy equally championship (link) [ verification needed ] [ non-primary source needed ] - ^ "Niccomo – Non-Invasive Continuous Cardiac Output Monitor". www.medis-de.com. medis. GmbH Ilmenau. Archived from the original on 17 October 2015. Retrieved 1 June 2015.
- ^ "Archived copy". Archived from the original on 24 May 2015. Retrieved 22 May 2015.
{{cite web}}: CS1 maint: archived copy as title (link) TEBCO OEM - ^ bomed.the states/ext-teb.html EXT-TEBCO
- ^ Krivitski NM (July 1995). "Theory and validation of access flow measurement by dilution technique during hemodialysis". Kidney International. 48 (1): 244–fifty. doi:10.1038/ki.1995.290. PMID 7564085.
- ^ Tanke RB, van Heijst AF, Klaessens JH, Daniels O, Festen C (Jan 2004). "Measurement of the ductal L-R shunt during extracorporeal membrane oxygenation in the lamb". Journal of Pediatric Surgery. 39 (i): 43–47. doi:ten.1016/j.jpedsurg.2003.09.017. PMID 14694369.
- ^ Casas F, Reeves A, Dudzinski D, Weber S, Lorenz M, Akiyama 1000, Kamohara K, Kopcak M, Ootaki Y, Zahr F, Sinkewich M, Foster R, Fukamachi K, Smith WA (2005). "Performance and reliability of the CPB/ECMO Initiative Frontwards Lines Prey Management System". ASAIO Journal. 51 (6): 681–85. doi:10.1097/01.mat.0000182472.63808.b9. PMID 16340350. S2CID 1897392.
- ^ Tessitore N, Bedogna V, Poli A, Mantovani W, Lipari Grand, Baggio E, Mansueto G, Lupo A (November 2008). "Adding access blood menstruation surveillance to clinical monitoring reduces thrombosis rates and costs, and improves fistula patency in the brusque term: a controlled accomplice study". Nephrology, Dialysis, Transplantation. 23 (11): 3578–84. doi:10.1093/ndt/gfn275. PMID 18511608.
- ^ van Loon M, van der Mark W, Beukers N, de Bruin C, Blankestijn PJ, Huisman RM, Zijlstra JJ, van der Sande FM, Tordoir JH (June 2007). "Implementation of a vascular access quality program improves vascular access care". Nephrology, Dialysis, Transplantation. 22 (6): 1628–32. doi:10.1093/ndt/gfm076. PMID 17400567.
- ^ (COstatus Archived 12 May 2015 at the Wayback Machine, Transonic System Inc. Archived 29 Oct 2008 at the Wayback Motorcar Ithaca, NY)[ non-primary source needed ]
- ^ Krivitski NM, Kislukhin VV, Thuramalla NV (July 2008). "Theory and in vitro validation of a new extracorporeal arteriovenous loop approach for hemodynamic assessment in pediatric and neonatal intensive intendance unit patients". Pediatric Critical Care Medicine. nine (4): 423–28. doi:10.1097/01.PCC.0b013e31816c71bc. PMC2574659. PMID 18496416.
- ^ Funk DJ, Moretti EW, Gan TJ (March 2009). "Minimally invasive cardiac output monitoring in the perioperative setting". Anesthesia and Analgesia. 108 (three): 887–97. doi:x.1213/ane.0b013e31818ffd99. PMID 19224798. S2CID 15891210.
- ^ Arheden H, Ståhlberg F (2006). "Blood flow measurements". In de Roos A, Higgins CB (eds.). MRI and CT of the Cardiovascular System (2nd ed.). Hagerstwon, Medico: Lippincott Williams & Wilkins. pp. 71–ninety. ISBN978-0-7817-6271-7.
- ^ Arheden H, Holmqvist C, Thilen U, Hanséus K, Björkhem M, Pahlm O, Laurin S, Ståhlberg F (May 1999). "Left-to-right cardiac shunts: comparison of measurements obtained with MR velocity mapping and with radionuclide angiography". Radiology. 211 (two): 453–58. doi:x.1148/radiology.211.2.r99ma43453. PMID 10228528.
- ^ Razavi R, Hill DL, Keevil SF, Miquel ME, Muthurangu Five, Hegde S, Rhode K, Barnett Thousand, van Vaals J, Hawkes DJ, Baker E (December 2003). "Cardiac catheterisation guided by MRI in children and adults with congenital heart illness". Lancet. 362 (9399): 1877–82. doi:x.1016/S0140-6736(03)14956-two. PMID 14667742. S2CID 25380774.
- ^ Kuehne T, Yilmaz S, Schulze-Neick I, Wellnhofer E, Ewert P, Nagel Due east, Lange P (August 2005). "Magnetic resonance imaging guided catheterisation for assessment of pulmonary vascular resistance: in vivo validation and clinical application in patients with pulmonary hypertension". Centre. 91 (8): 1064–69. doi:x.1136/hrt.2004.038265. PMC1769055. PMID 16020598.
- ^ Petzina R, Ugander M, Gustafsson 50, Engblom H, Sjögren J, Hetzer R, Ingemansson R, Arheden H, Malmsjö M (May 2007). "Hemodynamic effects of vacuum-assisted closure therapy in cardiac surgery: assessment using magnetic resonance imaging". The Periodical of Thoracic and Cardiovascular Surgery. 133 (v): 1154–62. doi:10.1016/j.jtcvs.2007.01.011. PMID 17467423.
- ^ Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK (Nov 2004). "Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Console written report". European Centre Journal. 25 (21): 1940–65. doi:ten.1016/j.ehj.2004.06.040. PMID 15522474.
- ^ Stewart J (2010). Calculus: Early Transcententals. Cengage Learning. pp. 565–66. ISBN9780538497909.
- ^ Sircar Due south (2008). Principles of Medical Physiology. Thieme. p. 237. ISBN978-one-58890-572-7.
- ^ Young DB (2010). Control of Cardiac Output. Morgan & Claypool Publishers. p. four. ISBN978-1-61504-021-6.
- ^ a b Vincent JL (2008). "Understanding cardiac output". Critical Care. 12 (four): 174. doi:10.1186/cc6975. PMC2575587. PMID 18771592.
- ^ Betts JG (2013). Beefcake & physiology. pp. 787–846. ISBN978-1938168130 . Retrieved xi Baronial 2014.
- ^ Levy MN, Berne RM (1997). Cardiovascular physiology (7th ed.). St. Louis: Mosby. ISBN978-0-8151-0901-3. [ page needed ]
- ^ Rowell, Loring B. (1993). Human cardiovascular command. Oxford: Oxford University Press. ISBN978-0-xix-507362-1. [ page needed ]
- ^ Braunwald E (1997). Heart disease: a textbook of cardiovascular medicine (5th ed.). Philadelphia: Saunders. ISBN978-0-7216-5666-3. [ page needed ]
- ^ Dhingra VK, Fenwick JC, Walley KR, Chittock DR, Ronco JJ (September 2002). "Lack of understanding betwixt thermodilution and fick cardiac output in critically ill patients". Chest. 122 (iii): 990–97. doi:10.1378/breast.122.3.990. PMID 12226045.
- ^ a b c d eastward f g Maceira AM, Prasad SK, Khan M, Pennell DJ (December 2006). "Reference right ventricular systolic and diastolic part normalized to historic period, gender and torso surface area from steady-country free precession cardiovascular magnetic resonance" (PDF). European Eye Journal. 27 (23): 2879–88. doi:10.1093/eurheartj/ehl336. PMID 17088316.
- ^ a b c d e f grand Maceira A (2006). "Normalized Left Ventricular Systolic and Diastolic Function past Steady Land Free Precession Cardiovascular Magnetic Resonance". Journal of Cardiovascular Magnetic Resonance. 8: 417–426. doi:10.1080/10976640600572889. (subscription required)
- ^ a b Normal ranges for heart rate are amongst the narrowest limits between bradycardia and tachycardia. See the Bradycardia and Tachycardia articles for more detailed limits.
- ^ a b "Normal Hemodynamic Parameters – Adult" (PDF). Edwards Lifesciences LLC. 2009.
- ^ WR Milnor: Hemodynamics, Williams & Wilkins, 1982[ ISBN missing ] [ page needed ]
- ^ BB Sramek: Systemic Hemodynamics and Hemodynamic Management, 2002, ISBN 1-59196-046-0[ folio needed ]
- ^ "Cardiac Output and Cardiac Index – What'south the deviation?". 13 December 2016. Retrieved 14 December 2018.
- ^ Boron WF (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1197. ISBN978-1-4160-2328-9. [ folio needed ]
- ^ "Arteriovenous oxygen departure". Sports Medicine, Sports Science and Kinesiology. Net Industries. 2011. Archived from the original on 12 June 2011. Retrieved 30 April 2011. [ unreliable medical source? ]
- ^ Iberti TJ, Fischer EP, Leibowitz AB, Panacek EA, Silverstein JH, Albertson TE (December 1990). "A multicenter written report of physicians' cognition of the pulmonary artery catheter. Pulmonary Avenue Catheter Study Grouping". JAMA. 264 (22): 2928–32. doi:10.1001/jama.264.22.2928. PMID 2232089.
- ^ Johnston IG, Jane R, Fraser JF, Kruger P, Hickling Thou (August 2004). "Survey of intensive care nurses' knowledge relating to the pulmonary artery catheter". Anaesthesia and Intensive Care. 32 (4): 564–68. doi:ten.1177/0310057X0403200415. PMID 15675218.
- ^ Alhashemi JA, Cecconi M, Hofer CK (2011). "Cardiac output monitoring: an integrative perspective". Critical Care. fifteen (two): 214. doi:10.1186/cc9996. PMC3219410. PMID 21457508.
External links [edit]
- Hemodynamics training for Junior Medical Staff
- The Gross Physiology of the Cardiovascular Organization
- The Determinants of Cardiac Output (online video)
- Basic Principles in Cardiac Physiology
Source: https://en.wikipedia.org/wiki/Cardiac_output


![{\displaystyle CO_{\text{[L/min]}}=SV_{\text{[L/beat]}}\times HR_{\text{[beats/min]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d9758ba05f9500a4ee209f0fb42f00e150e25a75)












![{\displaystyle BSA_{\mathrm {[m^{2}]} }=W_{\mathrm {[kg]} }^{0.425}\times H_{\mathrm {[cm]} }^{0725}\times 0.007184}](https://wikimedia.org/api/rest_v1/media/math/render/svg/00d2f209796a88f1509cc7ea78041537d6edfbaf)
![{\displaystyle SI_{\mathrm {[ml/beat/{m}^{2}]} }={\frac {SV_{\mathrm {[ml]} }}{BSA_{\mathrm {[m^{2}]} }}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4ee86865861d1119937669045bbc10f5568336f1)
![{\displaystyle CI_{\mathrm {[L/min/{m}^{2}]} }={\frac {CO_{\mathrm {[L/min]} }}{BSA_{\mathrm {[{m}^{2}]} }}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c0d5eea41bc4141c9a58bc2585202e3ddd2fcd36)
![{\displaystyle CI_{\mathrm {[L/min/{m}^{2}]} }=(SI_{\mathrm {[ml/beat/{m}^{2}]} }\times HR_{\mathrm {[bpm]} })/1000}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4ff4dbfb1013e029401ad5cfd6f4a16ab75c7ac5)


![{\displaystyle {\begin{aligned}{\text{Oxygen content of blood}}&=\left[{\text{haemoglobin}}\right]\left({\text{g/dL}}\right)\ \times \ 1.34\left({\text{mL}}\ {\ce {O2}}/{\text{g of haemoglobin}}\right)\\&\times \ {\text{saturation of blood}}\ \left({\text{percent}}\right)\ +\ 0.0032\ \times \ {\text{partial pressure of oxygen}}\left({\text{torr}}\right)\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/eba72e33526aeb0276cf5881842334b8ad841a3d)
0 Response to "Blood Flow to the Gi Tract Is Approximately What Percentage of the Total Cardiac Output?"
Post a Comment